SHARE
EV focused Lithium and Lead Batteries using Graphene
Written by Dr Oluwaseun John Dada
Dr Oluwaseun John Dada has worked for approx. 8 years or more on the synthesis, and tailoring of graphene and other nanoCarbons with phenomena publications.
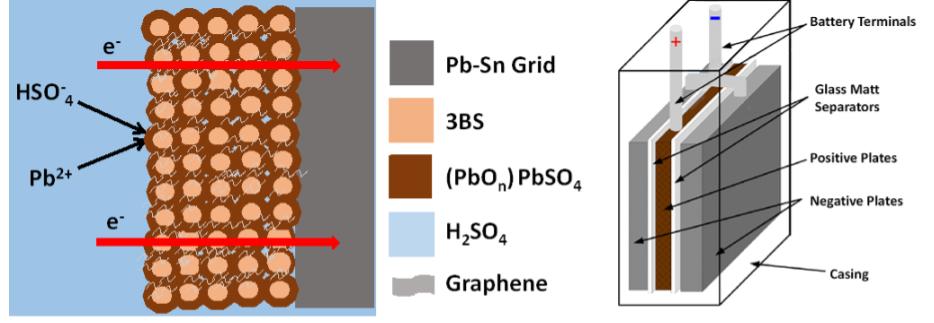
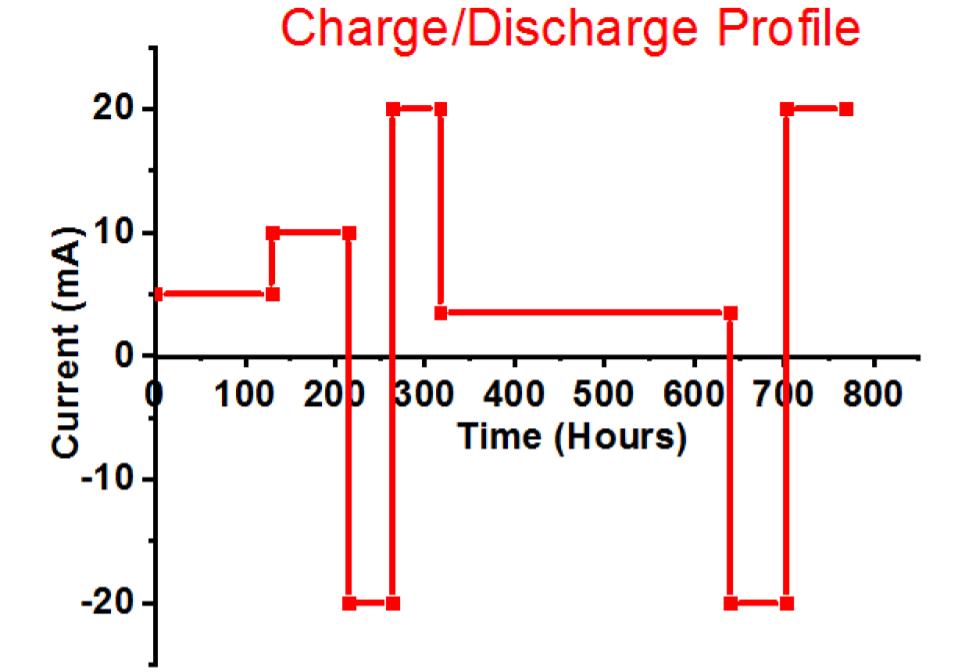
 Fig. 1. (a) Simple schematic of lead-acid battery, showing the positive active mass reaction and interpenetrated graphene additives within the formed PbO2.PbO.PbSO4 crust, (b) test cell design with two negative electrodes closing a positive electrode to make it the limit, and (c) charge-discharge profile.
The excellent and unique structural, surface, electrical and electronic characteristics of graphene and its variants hold great potential. Graphene oxide (GO) is a weak electrical conductor due to the presence of sp3 hybridized carbon, containing basal and edge located oxygen functionalities that hinder electron mobility. The conduction of electrons occurs due to the anionic nature of the GO functional surface; however, conducting properties are better restored when GO is reduced towards the original chemical structure of graphene.
GO reduction is not without structural defects and residual oxygen states. Reduced graphene (CCG) contains several bands consisting of sp3 carbons, sp2 carbons, including lone electron pairs and trapped water states in the π–band tail. Large sp2 domain sizes that are minimally interrupted by sp3 bonds are essential for obtaining exceptional electrical properties in reduced GO.
Both GO and CCG has resulted in a phenomenal increase in cycle life and discharge capacities in Lead Acid Battery (https://doi.org/10.1016/j.est.2019.04.004) shown in Fig. 2., while pristine Crystalline graphene with a low defect and planar characteristics causes phenomenal 300% increase in Lithium batteries (Dada, Oluwaseun John, High-Performance Lithium Battery Anodes from Non-Water based Graphene, April 8, 2019. Available at SSRN: https://ssrn.com/abstract=3368433.
Fig. 1. (a) Simple schematic of lead-acid battery, showing the positive active mass reaction and interpenetrated graphene additives within the formed PbO2.PbO.PbSO4 crust, (b) test cell design with two negative electrodes closing a positive electrode to make it the limit, and (c) charge-discharge profile.
The excellent and unique structural, surface, electrical and electronic characteristics of graphene and its variants hold great potential. Graphene oxide (GO) is a weak electrical conductor due to the presence of sp3 hybridized carbon, containing basal and edge located oxygen functionalities that hinder electron mobility. The conduction of electrons occurs due to the anionic nature of the GO functional surface; however, conducting properties are better restored when GO is reduced towards the original chemical structure of graphene.
GO reduction is not without structural defects and residual oxygen states. Reduced graphene (CCG) contains several bands consisting of sp3 carbons, sp2 carbons, including lone electron pairs and trapped water states in the π–band tail. Large sp2 domain sizes that are minimally interrupted by sp3 bonds are essential for obtaining exceptional electrical properties in reduced GO.
Both GO and CCG has resulted in a phenomenal increase in cycle life and discharge capacities in Lead Acid Battery (https://doi.org/10.1016/j.est.2019.04.004) shown in Fig. 2., while pristine Crystalline graphene with a low defect and planar characteristics causes phenomenal 300% increase in Lithium batteries (Dada, Oluwaseun John, High-Performance Lithium Battery Anodes from Non-Water based Graphene, April 8, 2019. Available at SSRN: https://ssrn.com/abstract=3368433.
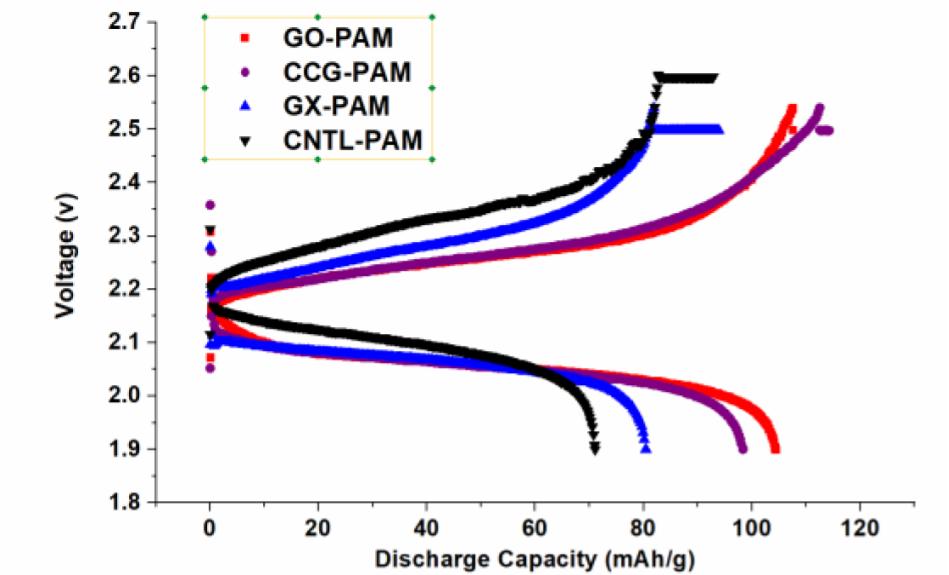
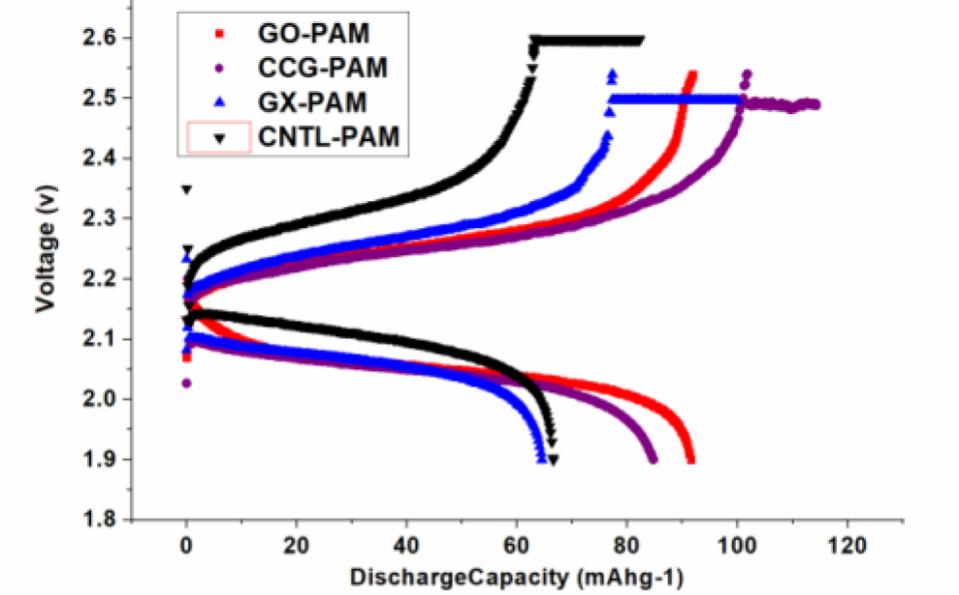
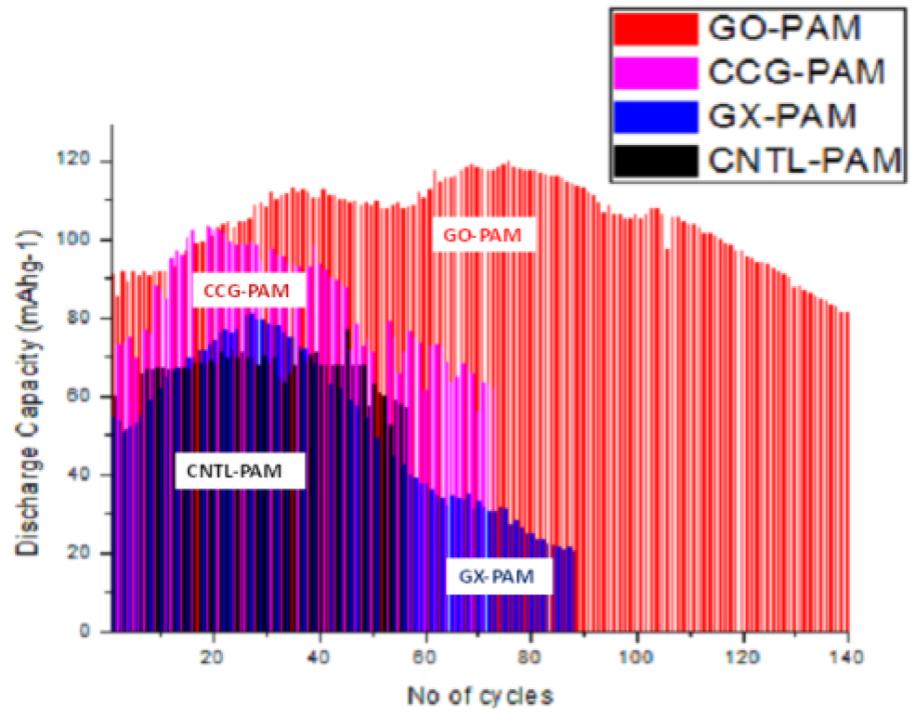
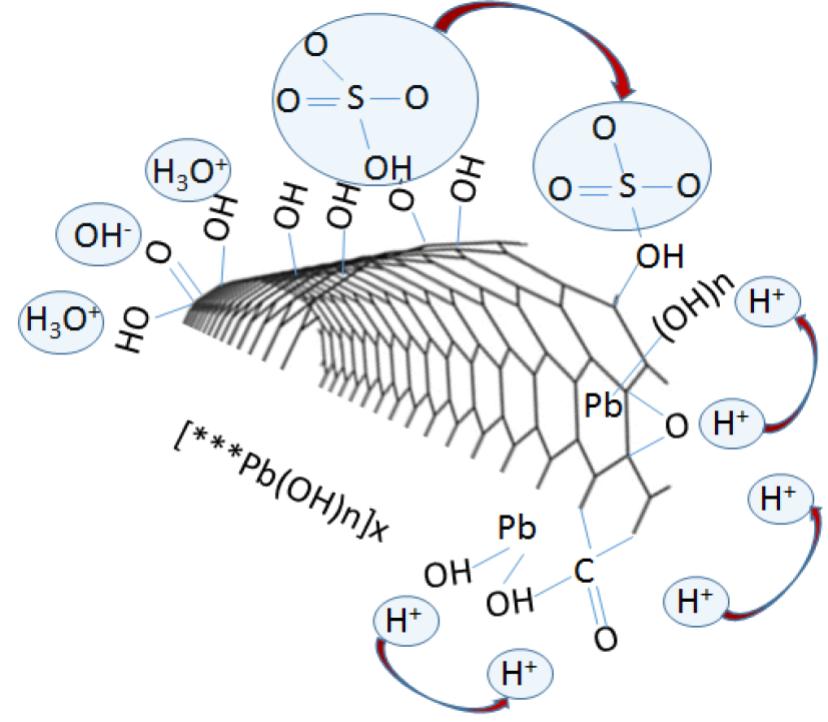
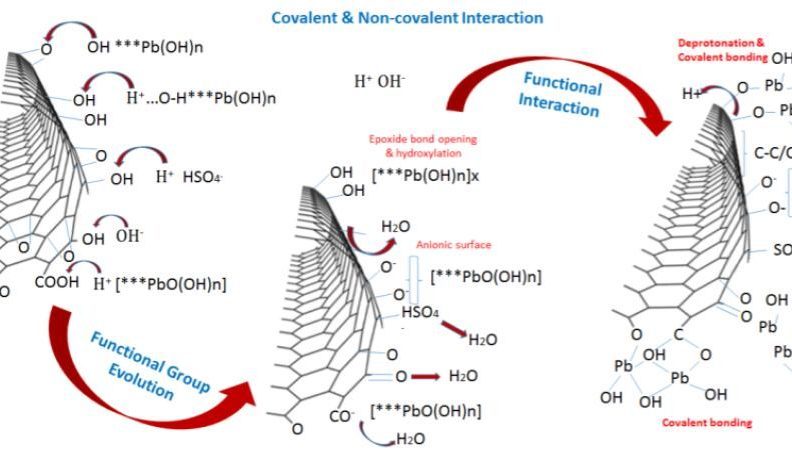 Fig. 3. (a) Mechanism of ion transfer and active sites nucleation during Pb salts and graphene interaction, and (b) Summary of active mass PbO2/Graphene bond interaction. Covalent and non-covalent interaction between graphene additives and the active material enhances electrode cohesion and bonding, as graphene additives participate in reactions enhancing active mass utilization.
For graphene-enhanced lithium battery, lithiation and de-lithiation are enhanced by the branching of the pristine graphene clusters and the preponderance of edge groups that the Li+ when transferred across the separator. This significantly increases the utilization of graphene sheet clusters within the electrode. The chemical purity of the pristine graphene enhances conductivity, and the planar characteristics and low defects increase the density of the graphene anode lithium battery plates. The more than 300% increase in anode capacity corresponds to almost 100% increase in the discharge capacity of the entire NMC//Gr battery. Reports have shown that commercial lithium batteries have about 3.5 times energy density with significantly higher cycle life compared to lead batteries (i.e. AGM), but the cost per amp/hrs is ~3 times higher. Scalable proof of concept is shown in Fig. 4.
Fig. 3. (a) Mechanism of ion transfer and active sites nucleation during Pb salts and graphene interaction, and (b) Summary of active mass PbO2/Graphene bond interaction. Covalent and non-covalent interaction between graphene additives and the active material enhances electrode cohesion and bonding, as graphene additives participate in reactions enhancing active mass utilization.
For graphene-enhanced lithium battery, lithiation and de-lithiation are enhanced by the branching of the pristine graphene clusters and the preponderance of edge groups that the Li+ when transferred across the separator. This significantly increases the utilization of graphene sheet clusters within the electrode. The chemical purity of the pristine graphene enhances conductivity, and the planar characteristics and low defects increase the density of the graphene anode lithium battery plates. The more than 300% increase in anode capacity corresponds to almost 100% increase in the discharge capacity of the entire NMC//Gr battery. Reports have shown that commercial lithium batteries have about 3.5 times energy density with significantly higher cycle life compared to lead batteries (i.e. AGM), but the cost per amp/hrs is ~3 times higher. Scalable proof of concept is shown in Fig. 4.
 Fig. 4. High-Performance Lithium batteries from Tailored NanoCarbons and Graphene scalable proof of concept.
If you know more about lithium batteries from nanocarbons like graphene, share your expertise with us and explore what ennomotive has to offer.
Join our community of engineers
Fig. 4. High-Performance Lithium batteries from Tailored NanoCarbons and Graphene scalable proof of concept.
If you know more about lithium batteries from nanocarbons like graphene, share your expertise with us and explore what ennomotive has to offer.
Join our community of engineers
EV focused Lithium and Lead Batteries with Stunning Performances using Tailored NanoCarbons like graphene
Technological demands in Hybrid Electric Vehicle (HEVs), renewable systems, and electrical storage systems, in addition to existing mature industrial process, recyclability and the low cost-per-energy, have extended industry interests and investments in Lithium & Lead batteries R&D. Several strategies have been used to improve the capacity and lifecycle of EV batteries, but only a few meet the routine design requirements of the current battery industry. Stunning battery performances have been achieved from using graphene’s tailored by Signature EcoSystems Technologies. For example, GO and CCG (Fig. 1.) has enhanced Lead-acid battery positive electrode by more than 41%, while novel 2D crystalline graphene gave the highest ever capacity increase in lithium battery anode, i.e. 300%, as proof of concept, scalable and within the mainstream of industrial design, rapidly marketable. (a) (b)
(c)

(d)
 Fig. 1. (a) Simple schematic of lead-acid battery, showing the positive active mass reaction and interpenetrated graphene additives within the formed PbO2.PbO.PbSO4 crust, (b) test cell design with two negative electrodes closing a positive electrode to make it the limit, and (c) charge-discharge profile.
The excellent and unique structural, surface, electrical and electronic characteristics of graphene and its variants hold great potential. Graphene oxide (GO) is a weak electrical conductor due to the presence of sp3 hybridized carbon, containing basal and edge located oxygen functionalities that hinder electron mobility. The conduction of electrons occurs due to the anionic nature of the GO functional surface; however, conducting properties are better restored when GO is reduced towards the original chemical structure of graphene.
GO reduction is not without structural defects and residual oxygen states. Reduced graphene (CCG) contains several bands consisting of sp3 carbons, sp2 carbons, including lone electron pairs and trapped water states in the π–band tail. Large sp2 domain sizes that are minimally interrupted by sp3 bonds are essential for obtaining exceptional electrical properties in reduced GO.
Both GO and CCG has resulted in a phenomenal increase in cycle life and discharge capacities in Lead Acid Battery (https://doi.org/10.1016/j.est.2019.04.004) shown in Fig. 2., while pristine Crystalline graphene with a low defect and planar characteristics causes phenomenal 300% increase in Lithium batteries (Dada, Oluwaseun John, High-Performance Lithium Battery Anodes from Non-Water based Graphene, April 8, 2019. Available at SSRN: https://ssrn.com/abstract=3368433.
Fig. 1. (a) Simple schematic of lead-acid battery, showing the positive active mass reaction and interpenetrated graphene additives within the formed PbO2.PbO.PbSO4 crust, (b) test cell design with two negative electrodes closing a positive electrode to make it the limit, and (c) charge-discharge profile.
The excellent and unique structural, surface, electrical and electronic characteristics of graphene and its variants hold great potential. Graphene oxide (GO) is a weak electrical conductor due to the presence of sp3 hybridized carbon, containing basal and edge located oxygen functionalities that hinder electron mobility. The conduction of electrons occurs due to the anionic nature of the GO functional surface; however, conducting properties are better restored when GO is reduced towards the original chemical structure of graphene.
GO reduction is not without structural defects and residual oxygen states. Reduced graphene (CCG) contains several bands consisting of sp3 carbons, sp2 carbons, including lone electron pairs and trapped water states in the π–band tail. Large sp2 domain sizes that are minimally interrupted by sp3 bonds are essential for obtaining exceptional electrical properties in reduced GO.
Both GO and CCG has resulted in a phenomenal increase in cycle life and discharge capacities in Lead Acid Battery (https://doi.org/10.1016/j.est.2019.04.004) shown in Fig. 2., while pristine Crystalline graphene with a low defect and planar characteristics causes phenomenal 300% increase in Lithium batteries (Dada, Oluwaseun John, High-Performance Lithium Battery Anodes from Non-Water based Graphene, April 8, 2019. Available at SSRN: https://ssrn.com/abstract=3368433.
(a)

(b)

(c)

(d)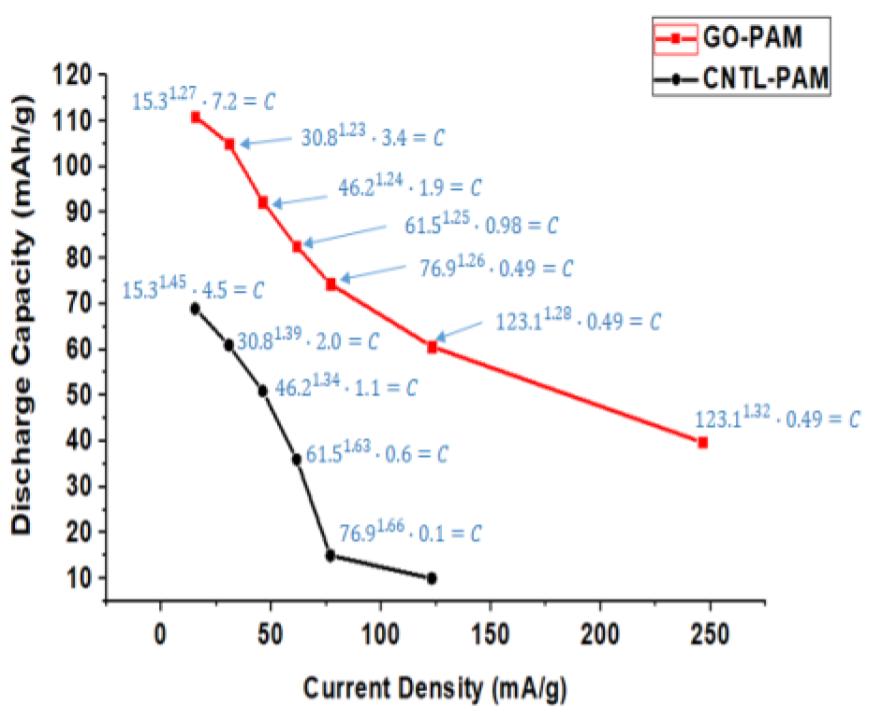
(a)

(b)
 Fig. 3. (a) Mechanism of ion transfer and active sites nucleation during Pb salts and graphene interaction, and (b) Summary of active mass PbO2/Graphene bond interaction. Covalent and non-covalent interaction between graphene additives and the active material enhances electrode cohesion and bonding, as graphene additives participate in reactions enhancing active mass utilization.
For graphene-enhanced lithium battery, lithiation and de-lithiation are enhanced by the branching of the pristine graphene clusters and the preponderance of edge groups that the Li+ when transferred across the separator. This significantly increases the utilization of graphene sheet clusters within the electrode. The chemical purity of the pristine graphene enhances conductivity, and the planar characteristics and low defects increase the density of the graphene anode lithium battery plates. The more than 300% increase in anode capacity corresponds to almost 100% increase in the discharge capacity of the entire NMC//Gr battery. Reports have shown that commercial lithium batteries have about 3.5 times energy density with significantly higher cycle life compared to lead batteries (i.e. AGM), but the cost per amp/hrs is ~3 times higher. Scalable proof of concept is shown in Fig. 4.
Fig. 3. (a) Mechanism of ion transfer and active sites nucleation during Pb salts and graphene interaction, and (b) Summary of active mass PbO2/Graphene bond interaction. Covalent and non-covalent interaction between graphene additives and the active material enhances electrode cohesion and bonding, as graphene additives participate in reactions enhancing active mass utilization.
For graphene-enhanced lithium battery, lithiation and de-lithiation are enhanced by the branching of the pristine graphene clusters and the preponderance of edge groups that the Li+ when transferred across the separator. This significantly increases the utilization of graphene sheet clusters within the electrode. The chemical purity of the pristine graphene enhances conductivity, and the planar characteristics and low defects increase the density of the graphene anode lithium battery plates. The more than 300% increase in anode capacity corresponds to almost 100% increase in the discharge capacity of the entire NMC//Gr battery. Reports have shown that commercial lithium batteries have about 3.5 times energy density with significantly higher cycle life compared to lead batteries (i.e. AGM), but the cost per amp/hrs is ~3 times higher. Scalable proof of concept is shown in Fig. 4.
 Fig. 4. High-Performance Lithium batteries from Tailored NanoCarbons and Graphene scalable proof of concept.
If you know more about lithium batteries from nanocarbons like graphene, share your expertise with us and explore what ennomotive has to offer.
Join our community of engineers
Fig. 4. High-Performance Lithium batteries from Tailored NanoCarbons and Graphene scalable proof of concept.
If you know more about lithium batteries from nanocarbons like graphene, share your expertise with us and explore what ennomotive has to offer.
Join our community of engineers